Our Technology
The Endocrine Activity Index (EAI) was developed at The University of Texas MD Anderson Cancer Center in Houston, TX by Dr. Fraser Symmans. The analytical and clinical validity of EAI has been established through multiple prospective-retrospective studies from randomized clinical trials.
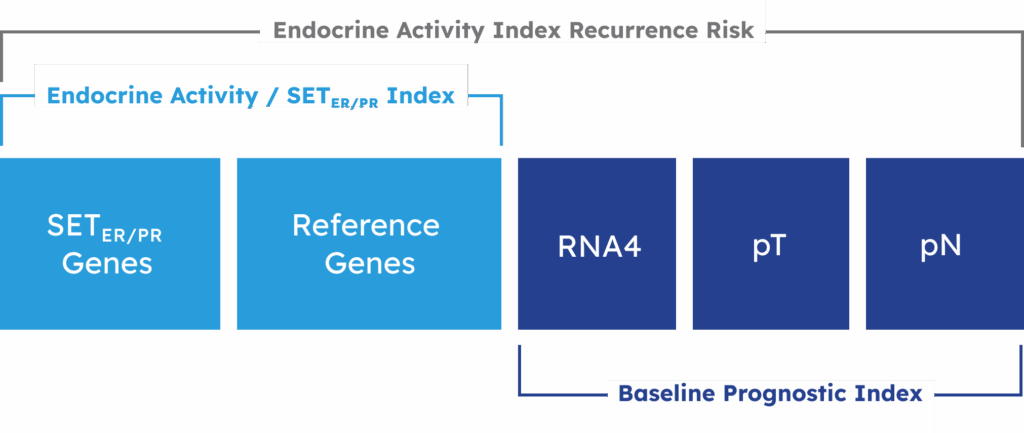
Components of Endocrine Activity Index
EAI is an 18-gene expression profile that measures transcriptional activity related to Estrogen Receptor (ER) and Progesterone Receptor (PR) in Stage II-III breast cancer tumor tissue to yield an Index Score.
To determine recurrence risk the EAI is combined with molecular subtype genes (RNA4) and clinical factors (pathologic tumor size (pT) and nodal status (pN)).
Our Technology
The Endocrine Activity Index is a gene expression profile test that measures transcriptional activity related to Estrogen Receptor (ER) and Progesterone Receptor (PR) in Stage II-III breast cancer tumor tissue to yield an Index Score. Gene expression is measured in a tumor sample from core biopsy or surgical resection.
Lead Inventor
Dr. W Fraser Symmans, MB.Chb.
Dr. Fraser Symmans is a Professor in the Departments of Pathology and Translational Molecular Pathology at University of Texas MD Anderson Cancer Center in Houston TX, where he practices Breast Surgical Pathology and Cytopathology and directs the Breast Cancer Pharmacogenomics Laboratory. He received his medical degree from the University of Auckland, New Zealand, completed his residency at Columbia University, New York, and fellowship at MD Anderson Cancer Center, Houston. Dr. Symmans joined the faculty of New York University Medical Center in 1993 and moved to MD Anderson Cancer Center in 2000.
Dr. Symmans serves on the Steering Committee for the Translational Breast Cancer Research Consortium, and is the Principal Investigator and Director of the Translational Research Program for the National Alliance for Clinical Trials cooperative group. He recently completed terms of voting membership of both, the Breast Cancer Steering Committee and the Correlative Sciences Review Committee for the National Clinical Trials Evaluation Program, National Cancer Institute. He is a funded researcher of the Breast Cancer Research Foundation (BCRF).
Dr. Symmans’ research is focused on breast cancer: neoadjuvant (pre-operative) treatment trials for evaluation of chemo- sensitivity and development of diagnostic tests to select the most effective treatments. Dr. Symmans is the lead inventor of the intellectual property underlying Delphi’s product line. Dr. Symmans holds founder shares in Delphi Diagnostics Inc. His employment with MD Anderson Cancer Center precludes him from being an employee, paid consultant or officer of Delphi Diagnostics.
Contact Us
Want to learn more about the Endocrine Activity Index (EAI) or Delphi Diagnostics?
Du L, Yau C, et al. Predicted sensitivity to endocrine therapy for stage II-III hormone receptor-positive and HER2-negative (HR+/HER2-) breast cancer before chemo-endocrine therapy. Ann Oncol. 2021;32(5):642-51. Epub 2021/02/23. doi: 10.1016/j.annonc.2021.02.011. PubMed PMID: 33617937
Patents: The following Delphi Diagnostics, Inc. products may be covered by one or more patents as indicated below. Notice is hereby provided under 35 U.S.C. §287(a) for the following products:
- SETER/PR The listed products and their use are the subject of US Patent Nos.: US 11,459,617
- SET2,3 The listed products and their use are the subject of US Patent Nos.: US 11,459,617

