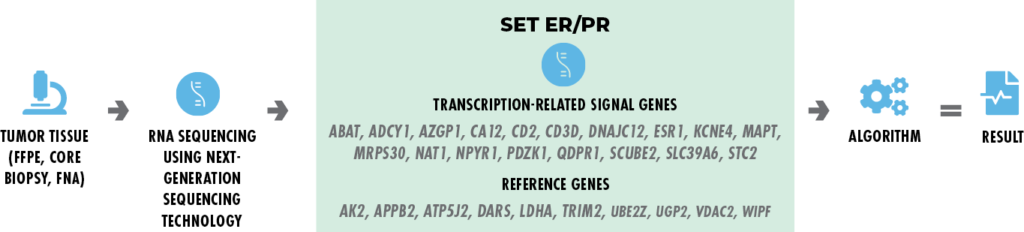
SETER/PR Index is an endocrine-related transcriptional gene signature. This is our core technology.
- Selected genes representing endocrine-receptors activity are measured in a breast cancer sample (Symmans et al. 2010) to yield the SETER/PR index score.
- Routinely preserved clinical tumor samples from core biopsy, fine-needle aspirate, or surgical resection can be used.
- Provides independent prognostic information in patients treated with adjuvant hormone therapies.
- A high score is associated with a good prognosis on endocrine therapies.
Endocrine Activity Index
(known in publications as SET2,3)
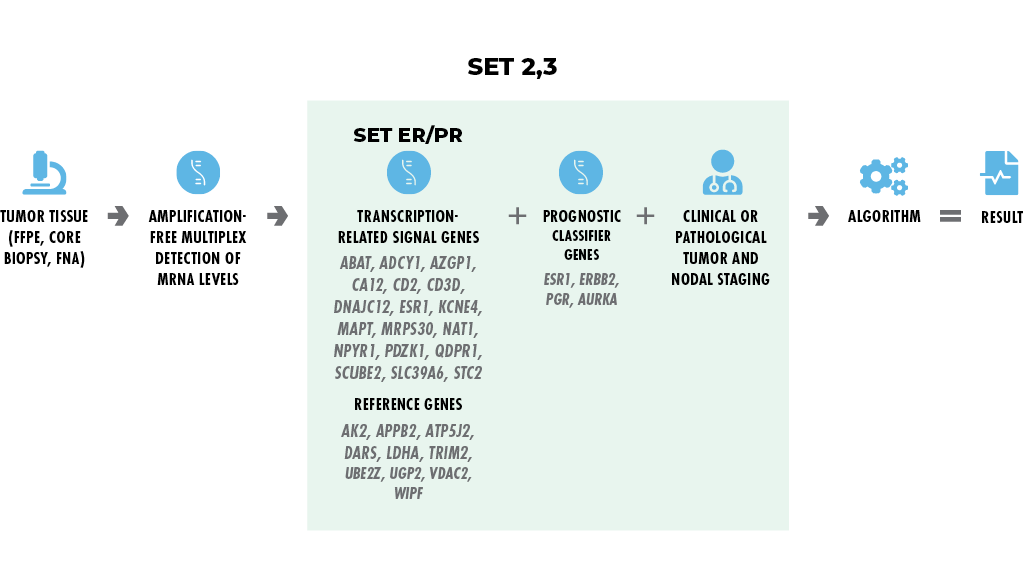
EAI is an assay that provides therapeutic insights on Stage II-III invasive breast cancer with nodal involvement.
- SETER/PR is determined from the tumor sample and the index score is adjusted for baseline prognosis using clinical factors (tumor size, nodal status) and molecular subtype genes (RNA4).
- EAI provides prognostic information independent of adjuvant and neoadjuvant chemotherapy response (Du et al. 2021).
- Currently implemented on a highly reproducible multiplex platform, validated across multiple laboratories (Bossuyt et al. 2021).
EAI4 Test
EAI4 is an assay that employs SETER/PR in the metastatic setting and is one of the only gene signatures that has shown prognostic efficacy in Stage IV breast cancers.
- Higher SETER/PR score was associated with longer progression-free and overall survival in Stage IV patients treated with endocrine therapy (Sinn et al. 2019).
- Implementation with advanced design on next generation RNAseq (Fu et al. 2021) allows interrogation of relevant mutational hotspots.
Patents
The following Delphi Diagnostics, Inc. products may be covered by one or more patents as indicated below:
Notice is hereby provided under 35 U.S.C. §287(a) for the following products:
| SETER/PRTM | The listed products and their use are the subject of US Patent Nos.: US 11,459,617 | |
| SET2,3 TM | The listed products and their use are the subject of US Patent Nos.: US 11,459,617 |