Today, many breast cancer patients experience adverse effects from routinely prescribed adjuvant therapies because current tests do not provide all the information required for clinicians to prescribe optimal personalized treatment decisions.
Delphi Diagnostics’s Endocrine Activity Index (EAITM) is a test that can provide actionable information to clinicians for prognosis Stage II-III breast cancer patients under standard of care therapy and for prediction of dose-dense chemotherapy benefit.
What is the Endocrine Activity Index?
Delphi’s Endocrine Activity Index (EAI) is a gene expression profiling (GEP) test that measures endocrine activity in tumors from patients with hormone receptor-positive HER2-negative Stage II-III breast cancer and uses an algorithm to predict survival outcomes for patients on standard-of-care therapy.
The EAI test has been shown in various studies1-4 to be:
- A consistent prognostic indicator for long-term outcomes in Stage II-III HR+HER- breast cancer patients.
- To provide independent prognostic information complementary to that contributed by other prognostic tests.
- Predictive for response to dose-intense chemotherapy (ddAC-T)
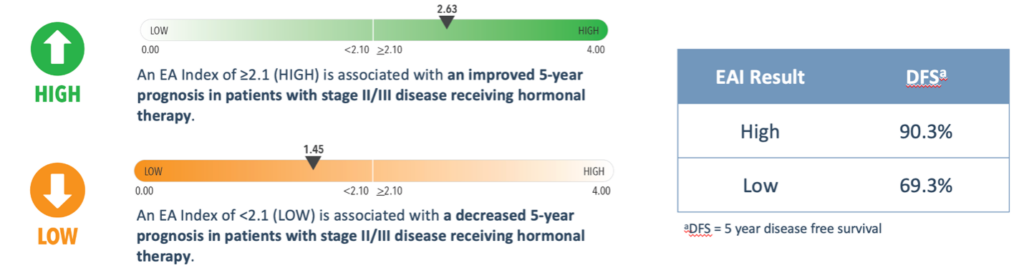
Patients with Stage II-III breast cancer receiving standard-of-care therapy and a High EAI Score, have been shown to have better 5-year disease-free survival (DFS) compared to patients with a Low EAI score.3
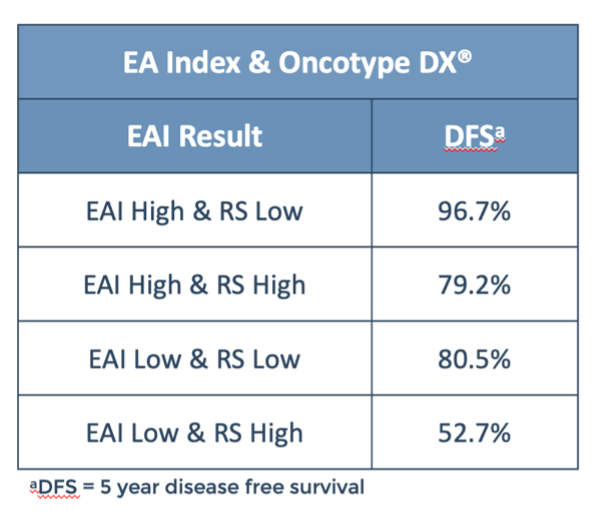
The EAI test has been shown to be both independent and add complementary information to other breast GEPs,1-3 including the 21-gene breast cancer recurrence score (RS).3
The use of these two test results together may provide additional information to inform personalized management decisions in patients with Stage II-III breast cancer3.
Delphi’s Early Access Program:
The EAI test is being offered to breast cancer patients through a select number of physicians who are participating in our Early Access Program. Interested in offering EAI to your patients? Please contact us at medicalaffairs@delphi-diagnostics.com
EAI is being offered by Protean BioDiagnostics Inc.
References:
- Du L, Yau C, et al. Predicted sensitivity to endocrine therapy for stage II-III hormone receptor-positive and HER2-negative (HR+/HER2-) breast cancer before chemo-endocrine therapy. Ann Oncol. 2021;32(5):642-51. Epub 2021/02/23. doi: 10.1016/j.annonc.2021.02.011. PubMed PMID: 33617937
- Suman VJ, Du L, Hoskin T, Anurag M, Ma C, Bedrosian I, Hunt KK, Ellis MJ, Symmans WF. Evaluation of Sensitivity to Endocrine Therapy Index (SET2,3) for Response to Neoadjuvant Endocrine Therapy and Longer-Term Breast Cancer Patient Outcomes (Alliance Z1031). Clin Cancer Res. 2022 Aug 2;28(15):3287-3295. doi: 10.1158/1078-0432.CCR-22-0068. PMID: 35653124; PMCID: PMC9357183
- Speers CW, Symmans WF, Barlow WE, Trevarton A, The S, Du L, Rae JM, Shak S, Baehner R, Sharma P, Pusztai L, Hortobagyi GN, Hayes DF, Albain KS, Godwin A, Thompson A. Evaluation of the Sensitivity to Endocrine Therapy Index and 21-Gene Breast Recurrence Score in the SWOG S8814 Trial. J Clin Oncol. 2023 Jan 17:JCO2201499. doi: 10.1200/JCO.22.01499. PMID: 36649570
- Metzger, Otto et al. Measurement of endocrine activity (SET2,3) related to prognosis and prediction of benefit from dose-dense (DD) chemotherapy in estrogen receptor-positive (ER+) cancer: CALGB 9741 (Alliance). 2022 American Society of Clinical Oncology Annual Meeting, Chicago, IL. June 3-7, 2022. https://meetings.asco.org/abstracts-presentations/208274 and 2023 San Antonio Breast Cancer Symposium, invited presentation.
Patents
The following Delphi Diagnostics, Inc. products may be covered by one or more patents as indicated below:
Notice is hereby provided under 35 U.S.C. §287(a) for the following products:
| SETER/PRTM | The listed products and their use are the subject of US Patent Nos.: US 11,459,617 | |
| SET2,3 TM | The listed products and their use are the subject of US Patent Nos.: US 11,459,617 |