Endocrine Activity Index: Recurrence Risk
The Endocrine Activity Index Recurrence Risk (EAI RR) has been shown to be a consistent prognostic indicator for long-term outcomes in Stage II-III breast cancer patients.

Refine your patients prognosis
Multiple clinical trials have shown that the EAI RR test is a consistent prognostic indicator for long-term outcomes in Stage II-III breast cancer patients. EAI RR is also independent from other prognostic tests, allowing you to further understand your patient’s recurrence risk.
Clinical Evidence
EAI has been clinically validated in multiple clinical trials with >4600 patients with consistent results as a prognostic indicator for long term outcomes (DFS, EFS, DRFS, iDFS, DRFI) in Stage II-III breast cancer patients.
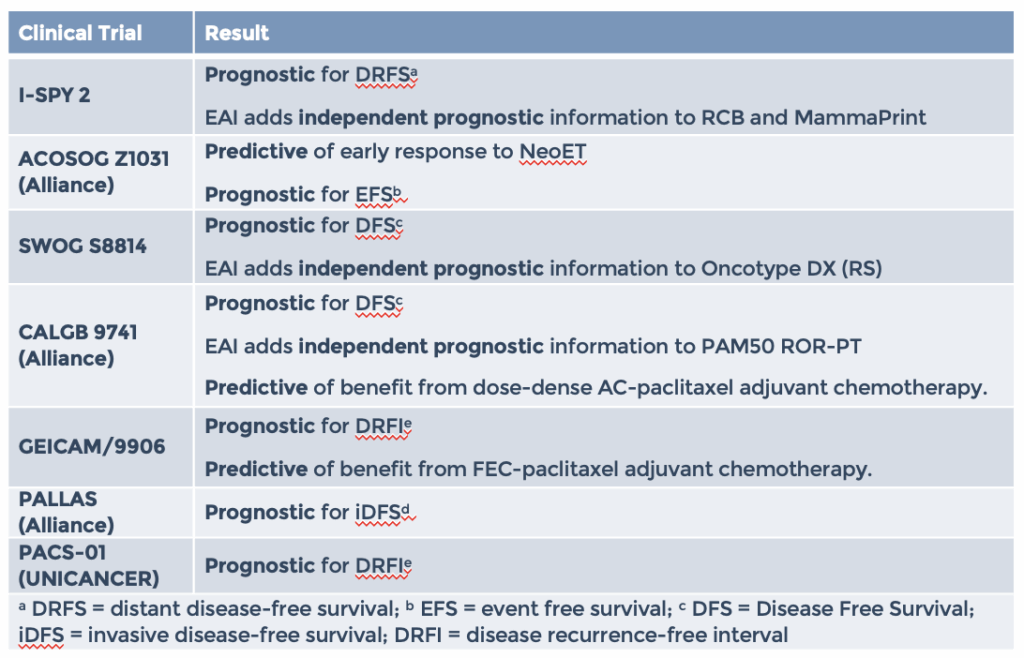
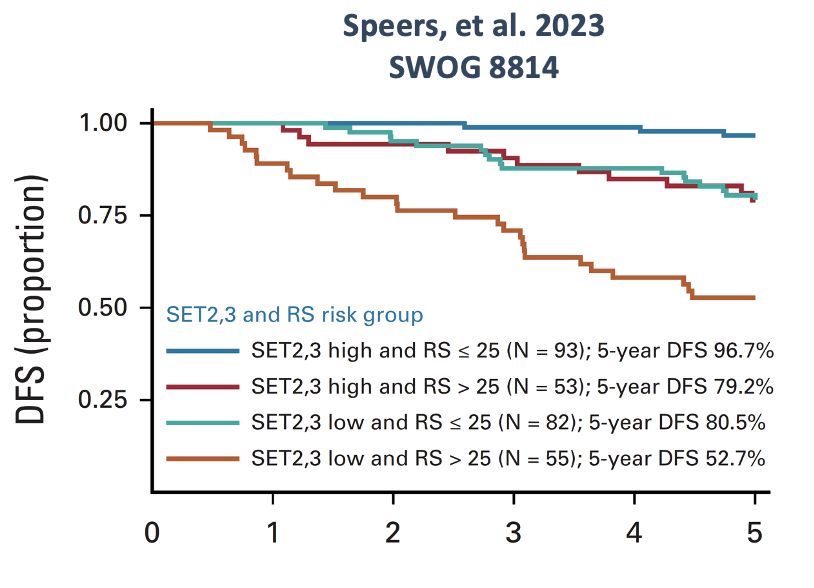
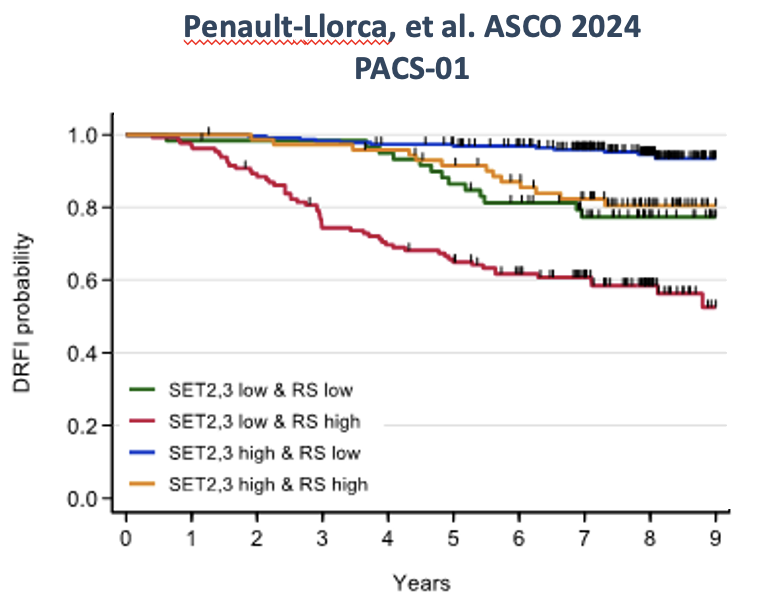
EAI test has been shown to be independent from OncotypeDX, ROR-PT and MammaPrint in multivariate models. Results of two independent validation studies (shown below) totaling over 900 patients demonstrate that the EAI RR provides independent and additive prognostic information to the 21-gene Recurrence Score. Findings suggest that results from both tests may be useful when counseling women about the risks of cancer recurrence and the most appropriate management strategy.
Visit our publications page for links to the publications mentioned above
Report Overview
EAI RR combines the genomic measurement of endocrine activity with baseline risk factors to determine breast cancer prognosis. This information is provided in a simple easy to interpret format on our clinician report.

Contact Us
Want to learn more about the Endocrine Activity Index (EAI) or Delphi Diagnostics?
1.Speers CW, Symmans WF, Barlow WE, Trevarton A, The S, Du L, Rae JM, Shak S, Baehner R, Sharma P, Pusztai L, Hortobagyi GN, Hayes DF, Albain KS, Godwin A, Thompson A. Evaluation of the Sensitivity to Endocrine Therapy Index and 21-Gene Breast Recurrence Score in the SWOG S8814 Trial. J Clin Oncol. 2023 Jan 17:JCO2201499. doi: 10.1200/JCO.22.01499. Epub ahead of print. PMID: 36649570.
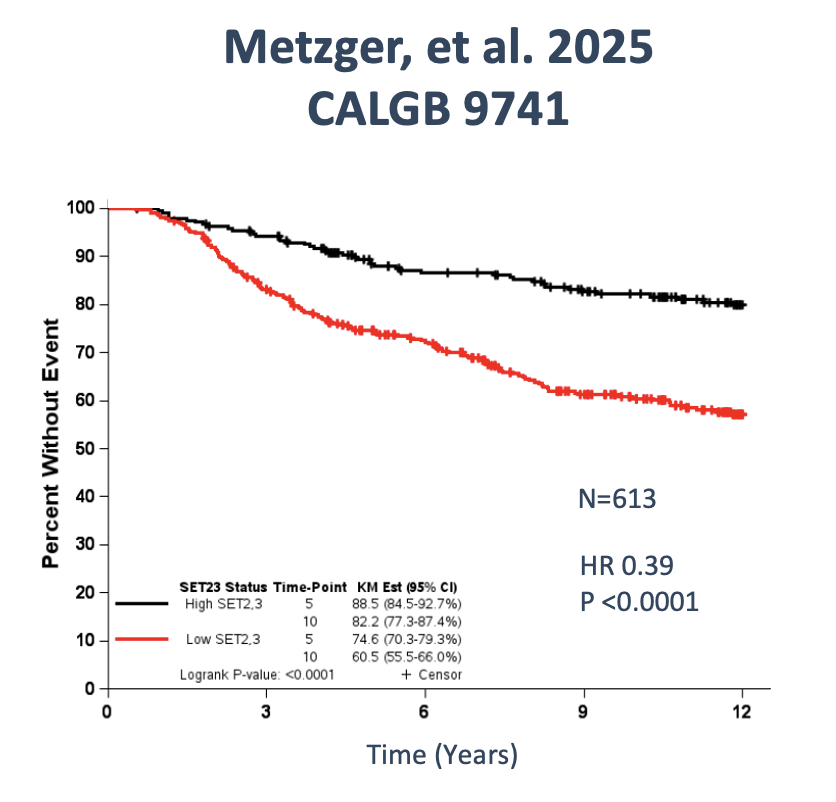
2.Metzger O, Ballman K, Campbell J, Liu MC, Ligibel JA, Chen E, Symmans F. Adjuvant dose-dense chemotherapy in hormone receptor-positive breast cancer. Journal of Clinical Oncology 2024
3.Penault-Llorca F, et al. Combination of predicted sensitivity to endocrine therapy (SET2,3 index) and the Recurrence Score® in node-positive breast cancer: independent validation in the PACS-01 trial. 2024 American Society of Clinical Oncology Annual Meeting, Chicago, IL. May 31-Jun 4, 2024. J Clin Oncol 42, 2024 (suppl 16; abstr 565), DOI: 10.1200/JCO.2024.42.16_suppl.565
4.Metzger O, et al. Analysis of the sensitivity to endocrine therapy (SET) assay in the PALLAS adjuvant trial of palbociclib in HR+/HER2- breast cancer (ABCSG-42/AFT-05/BIG-14-13). 2024 American Society of Clinical Oncology Annual Meeting, Chicago, IL. May 31-Jun 4, 2024. J Clin Oncol 42, 2024 (suppl 16; abstr 538), DOI: 10.1200/JCO.2024.42.16_suppl.538
